K.
pneumoniae is a species of bacteria that is found frequently in the
environment and in people; it is estimated that over 1/3 of the world’s
population is colonized by K. pneumoniae. In
individuals with a healthy immune system, the level of bacteria is controlled, and illness does not occur. However, in immunocompromised and
already-ill people, the bacteria can cause severe infections. Klebsiella are
the third leading cause of hospital-acquired infections in the United States,
with alarmingly high mortality rates: K. pneumoniae pneumonia
can cause mortality in up to 50% of patients, and bloodstream infections can
cause mortality in 20-30%. Equally concerning is the rising level of antibiotic
resistance found in K. pneumoniae. This
makes them increasingly difficult to treat.
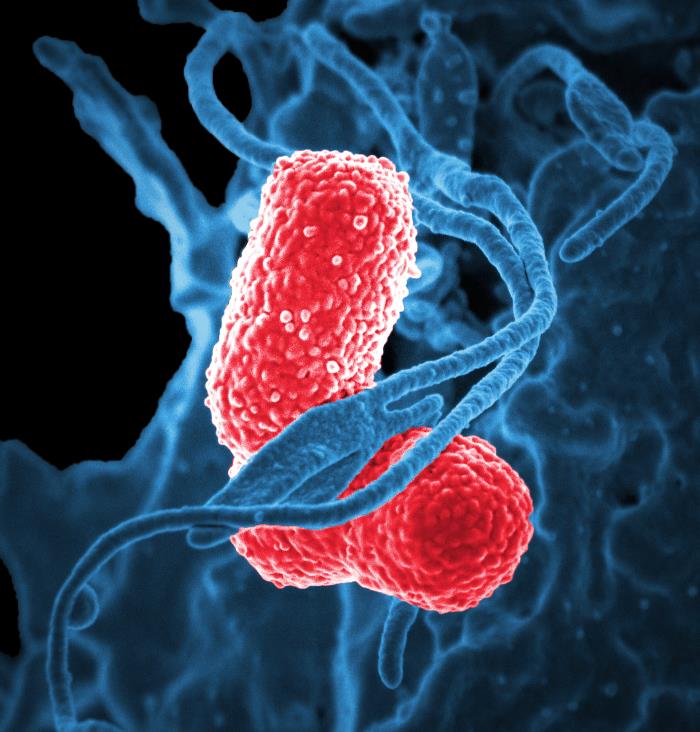 |
| K. pneumoniae (red). CDC's Public Health Image Library. Image # 18170; photo credit: NIAID. |
These antibodies open the possibility for their use as therapeutics. Giving pre-made antibodies to patients has already been established as an effective strategy to treat or prevent a number of infectious diseases, such as rabies, diptheria, tetanus, hepatitis B, and botulism. In the case of these infections, antibodies that are highly specific to the pathogen of interest have been made and used. However, the identification of these broadly neutralizing antibodies opens the door for a new opportunity. Giving a patient "universal" antibodies could help fight a variety of infections without even necessarily identifying the causative agent, which can be difficult and time-consuming in the face of a life-threatening infection. While it is a long process from antibody identification to the approved use of an antibody as a therapy in patients, this discovery provides researchers direction for the path ahead. Therapeutic advances that use alternative strategies to inhibit and kill pathogens are of the utmost importance in the current age of antibiotic resistance. Antibodies, instead of just antibiotics, that can fight disease will be one of the important tools in our arsenal against the ever-evolving microbes.