The annoying buzz of pesky little mosquitoes. At this time of year, the sound can be heard in backyards all around the world. Due to emerging threats like Zika and Dengue virus, people are becoming more and more familiar with the harm these little devils can cause. While vaccines and treatments for many mosquito-borne illnesses remain elusive, some researchers are placing new hope in other micro-organisms who may be able to play a role in preventing these dangerous diseases.
Previous work focused on malaria and its vector, the anopheline mosquitoes, has implicated micro-organisms, such as bacteria or fungi, within the mosquito to have an effect on the mosquito's ability to transmit the disease. Numerous studies in both Anopheles stephensi and Anopheles gambiae have shown that microbes in their midguts can influence the malarial parasite's ability to develop and transmit to new hosts. This is also not a new concept in the Aedes mosquitoes, the vectors for many of the mosquito-borne viruses. Wolbachia bacteria have been successfully introduced multiple times into these mosquitoes to prevent infection in the past.
Most previous studies have focused on introducing microbes to adult mosquitoes. In the field, this naturally presents challenges with application as adult mosquitoes are constantly migrating from place to place. However, recent work highlights that similar strategies could be used on mosquito larvae to influence their ability to transmit disease as adults. Larvae are much more targetable, due to the relative ease of identifying larval development sites.
In the latest study of the mosquito microbiota and its effects on vectorial capacity (the ability of a vector, like the mosquito, to transmit a particular disease), researchers found that differences in bacterial colonization of larvae could have an effect on the adult mosquito's traits. This means that by changing the bacteria larvae are exposed to, you could influence their ability to transmit disease as adults.
While there are many environmental concerns that will need to be addressed before a strategy altering the bacterial make-up of mosquito breeding sites, this new study and the growing body of work focusing on the mosquito microbiome's effects on vectorial capacity offer hope for new strategies to control disease spread. Perhaps some day we will be able to add a simple pellet of bacteria to a pool of water with mosquito larvae and prevent all the subsequent adults from transmitting disease. This may seem like a far-fetched dream today, but with continued research, it could one day be a reality.
My goal is to write about cutting-edge biology and fill my readers in on the latest research, mainly in the realm of infectious diseases.
Thursday, August 31, 2017
Monday, July 31, 2017
New diagnostic method may help fight antibiotic resistance
Coughing, sneezing, runny nose, wheezing; respiratory tract infections are extremely common, with the average American adult enduring two to four each year. Typically, the symptoms last for seven to ten days while you struggle through, and then you get better. If you go to a doctor with these symptoms, they will likely prescribe you a broad-range antibiotic. This may sound fine, but there is a big issue with that: not all respiratory tract infections are caused by a bacterial infection. Over-use of antibiotics has been leading to increased antibiotic resistance for decades. The only way to prevent the over-prescribing of antibiotics for these respiratory tract infections is to determine the cause of each infection. Unfortunately, current technologies for diagnosing these infections are not fast and specific enough to allow timely and proper diagnosis. New technology has emerged that may help with the diagnosis and cut down on the over-use of antibiotics.
When exposed to different pathogens, the body's immune system responds in different ways. A virus, for example, causes cells to react in a different way than a bacterium. The cells of the immune system that can be found in the blood can be profiled to understand what type of infection they are fighting. This has been done by studying the messenger RNAs (mRNAs), also known as transcripts, found within monocytes, a specific subset of immune cells. mRNAs lead to the proteins being made by a cell and often play key roles in regulating the activation of pathways involved in an immune response. A recent study identified ten different mRNAs that could be used to determine if the body was responding to a bacterial or a viral respiratory tract infection. A more recent study further validated these ten mRNAs by confirming their use in 94 hospitalized adults with respiratory tract infections and identified even more mRNAs that could differentiate a bacterial infection from a viral infection. This allows for more appropriate use of antibiotics in these patients and avoids the potential over-use of antibiotics that threatens their effectiveness.
As more and more antibiotic-resistant infections emerge, it is becoming more important than ever to safeguard our potent antibiotics by only using them when necessary. When antibiotics are used, they kill off whatever bacteria are susceptible to their effects, leaving behind only those that are resistant. This helps select for antibiotic-resistant bacteria within a population. The over-use of antibiotics has sped this natural selection process, allowing for the rapid development of resistance even to the newest antibiotics. Using technologies such as this transcript profiling of immune cells will help slow the selection process by ensuring antibiotics are only introduced when they can be of help, allowing our antibiotics to maintain their usefulness longer before resistance develops.
The technology to profile transcripts of immune cells in the blood has the opportunity for application far beyond the identification of bacterial versus viral respiratory infection. Individual pathogens themselves can produce unique immune profiles that could one day be categorized using similar methods. With the standard diagnostics of the past, it is nearly impossible to diagnose a bacterial or viral infection unless the bacteria can be cultured or the virus can be isolated. Knowing the unique transcript profile induced by a pathogen could someday allow for molecular diagnosis of a number of pathogens without the need to culture the bacteria or isolate the virus, allowing for an even more significant reduction in antibiotic over-use. This would also provide more rapid diagnosis; transcript analysis could be performed in a matter of hours, while the diagnostics of the past frequently require days. With emerging technologies, these goals become even more achievable every day, and we may soon see a time when antibiotics are only used for infections confirmed to be caused by a susceptible bacteria.
When exposed to different pathogens, the body's immune system responds in different ways. A virus, for example, causes cells to react in a different way than a bacterium. The cells of the immune system that can be found in the blood can be profiled to understand what type of infection they are fighting. This has been done by studying the messenger RNAs (mRNAs), also known as transcripts, found within monocytes, a specific subset of immune cells. mRNAs lead to the proteins being made by a cell and often play key roles in regulating the activation of pathways involved in an immune response. A recent study identified ten different mRNAs that could be used to determine if the body was responding to a bacterial or a viral respiratory tract infection. A more recent study further validated these ten mRNAs by confirming their use in 94 hospitalized adults with respiratory tract infections and identified even more mRNAs that could differentiate a bacterial infection from a viral infection. This allows for more appropriate use of antibiotics in these patients and avoids the potential over-use of antibiotics that threatens their effectiveness.
As more and more antibiotic-resistant infections emerge, it is becoming more important than ever to safeguard our potent antibiotics by only using them when necessary. When antibiotics are used, they kill off whatever bacteria are susceptible to their effects, leaving behind only those that are resistant. This helps select for antibiotic-resistant bacteria within a population. The over-use of antibiotics has sped this natural selection process, allowing for the rapid development of resistance even to the newest antibiotics. Using technologies such as this transcript profiling of immune cells will help slow the selection process by ensuring antibiotics are only introduced when they can be of help, allowing our antibiotics to maintain their usefulness longer before resistance develops.
The technology to profile transcripts of immune cells in the blood has the opportunity for application far beyond the identification of bacterial versus viral respiratory infection. Individual pathogens themselves can produce unique immune profiles that could one day be categorized using similar methods. With the standard diagnostics of the past, it is nearly impossible to diagnose a bacterial or viral infection unless the bacteria can be cultured or the virus can be isolated. Knowing the unique transcript profile induced by a pathogen could someday allow for molecular diagnosis of a number of pathogens without the need to culture the bacteria or isolate the virus, allowing for an even more significant reduction in antibiotic over-use. This would also provide more rapid diagnosis; transcript analysis could be performed in a matter of hours, while the diagnostics of the past frequently require days. With emerging technologies, these goals become even more achievable every day, and we may soon see a time when antibiotics are only used for infections confirmed to be caused by a susceptible bacteria.
Thursday, June 29, 2017
More vaccination victories needed in the meningitis fight
Vaccinations have been proven time and time again to prevent
disease and improve health outcomes. All around the world, vaccines have been
deployed to deal with illnesses as common as the flu and as deadly as Ebola. Meningitis
is another disease for which vaccination has become a major priority. The
“kissing disease,” at it is sometimes called, has made a number of appearances
on college campuses across the United States. While incidence in the U.S.
remains quite low, at 0.3-4 cases per 100,000 persons, incidence can be as high
as 1 case per 100 persons in the “meningitis belt” of Africa, where epidemics
occur with regularity.
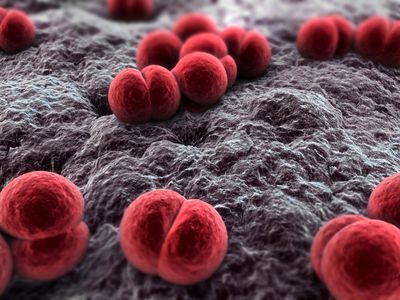 |
| Neisseria meningitidis, the bacterium responsible for meningitis. Image from Bioquell.com |
Infection with the bacterium Neisseria meningitidis, the major cause of meningitis, often goes
unnoticed. The bacteria take up residence within the nasal cavity, where they can
stay without causing disease in a carrier individual. However, in approximately
1-5% of people exposed to the bacteria, invasive disease occurs, and the
bacteria enter the bloodstream, leading to life-threatening disease.
Symptoms of meningitis typically begin almost immediately,
just one day after infection, and include flu-like symptoms of fever, headache,
and stiffness. Because the bacteria enter the bloodstream, any organ or tissue
can become infected and impaired. Despite years of research, mortality rates
continue to range from 10-15%, even in developed countries, with rates above
20% in the developing world. Even for those who survive the invasive disease
stage, meningitis causes lasting impairments in 19% of patients, with
neurological disabilities, seizures, hearing or visual loss, and cognitive
impairment being classical manifestations. The rapidity of disease progression,
along with the high mortality rate, make meningitis a prime disease target for
vaccination.
The first vaccines against meningitis were developed in the
1970s. Unfortunately, these early vaccines lacked the ability to maintain
long-lasting immunity against the bacteria. In the late 1990s, alterations were
made in the vaccine components, allowing for the elicitation of an
immunological memory response that would be effective to protect young children
into their adult years and would even help reduce the rates of carriage of the
bacteria in the nasal cavity. While this was great news for the prevention of
meningitis, challenges still remained. The bacteria that cause disease can
belong to any of 6 different serogroups, meaning that immunity to one serogroup
will not necessarily provide protection from another. This requires
differential targeting of all 6 serogroups to truly prevent disease.
 |
| Image from the Meningitis Vaccine Project |
Researchers have addressed this challenge by producing different
vaccines for use in specific parts of the world where each serogroup is
problematic. In the meningitis belt of Africa, for example, serogroup A has
historically been the cause of epidemics. To wipe out these epidemics, a mass
vaccination campaign was begun in 2010; the Meningitis Vaccine Project produced
and provided vaccines against N.
meningitidis serogroup A for over 217 million people in 17 different
countries. Thanks to these vaccines, epidemics linked to the serogroup A
bacteria have been eliminated.
Unfortunately, when one serogroup is removed, a niche opens
up for another. Just last month, the CDC announced that a small epidemic in
Liberia had been caused by the N.
meningitidis serogroup C bacteria. Nigeria and Niger have also reported
outbreaks of this serogroup. Luckily, in the case of Liberia, the country’s
response time was extremely rapid. Thanks to the health system improvements
made during the Ebola outbreak, Liberia now has a robust case detection and
monitoring system. Other countries in the area, however, are not nearly as
advanced and could suffer a severe epidemic if serogroup C moves in with force.
Great strides have been made in the fight against meningitis
outbreaks. However, the complexity of the group of bacteria responsible for the
disease leaves a number of challenges in place that must be overcome. The ideal
solution would be the introduction of a vaccine that combined pieces from each
bacteria serogroup to produce an immune response in patients that would protect
from all six serogroups at the same time. While some quadrivalent vaccines
already exist, which provide protection against four of the six serogroups,
these vaccines have only been recommended for use in the U.S. for adolescents
entering college. Protection from this vaccine only lasts 2-5 years in adults,
making it less than ideal for deployment in rural areas where boosting is not a
viable option, such as Africa. Advances in vaccine technology may help improve
the longevity of protection, making multivalent vaccination a more robust
solution to the meningitis problem. Until then, rapid case detection and
monitoring capabilities, such as those displayed in Liberia, will be the key to
keeping meningitis epidemics in check as they arise. Between vaccine and
monitoring advances, meningitis epidemics may one day become a thing of the
past.
Sunday, May 21, 2017
The Stressed-out Microbiome
Stress. It's something we encounter every day. People teach workshops and write books on how to deal with it. We spend countless hours and dollars trying to avoid it. But it's still there. Stress is as much a constant in our world today as the air around us. We all know that stress can have negative impacts on mental well-being, but we are just beginning to realize the effects stress can have on other bodily systems, and the effect these systems can have on our ability to deal with stress. Recent research has shown that the microbiome can be significantly affected by and play a major role in our response to stress.
The microbiome is the group of microbes (bacteria, fungi, archaea, etc.) that colonize a particular area of the body. The gut microbiome has been a population of intense research for quite a few years now. With increasing technology, we've begun to learn more and more about this population. We know that at homeostasis, each person has a specific microbial population that makes up their gut microbiome. This helps keep us healthy, keeping our digestive system running smoothly and ensuring successful processing of the food we eat. But in times of disease or stress, the microbiome in the gut can change dramatically, causing symptoms from loss of appetite to ulcers.
How exactly does stress change what's living in your intestines? It's all because of the gut-brain communications. This is achieved through neural projection pathways, neuroendocrine signaling, and entero-endocrine signaling, among other pathways. Your body uses messaging through the neurons and small chemical messengers in the form of endocrine signals to communicate over long distances, such as from the brain to the gut. In return, the gut uses the same mechanisms to send feedback to the brain. The microbes in the gut, however, can also produce chemical messages that get sent to the brain. When your body experiences stress, different chemical signals are sent to the gut; this can, in turn, cause some microbes to die and allow other microbes to take their place. The change in the composition of microbes can then change the chemical signals sent back to the brain. In this way, there is an intimate connection between the signalling input from the brain and the feedback the brain receives. These signals are essential to support mood, higher cognitive function, and behavior.
Work in animal models has highlighted the influence of stress on the gut microbiome and the subsequent influence of the microbiome on behaviors. For example, when mice are raised in a germ-free environment (no microbiome), the mice show reduced anxiety-like behaviors as compared to mice that are not raised in a germ-free environment. It has also been shown that the microbiome of the mice has an influence on the development of the amygdala, a region of the brain that plays a vital role in controlling behavioral and physiological responses to stress stimuli. The germ-free mice showed increased amygdala volume, suggesting an increased ability to handle stress. Additional studies have found that early-life stress can change an animal's microbiome for the rest of its life and that microbiome transplantation from depressed rats to healthy rats can lead to anxiety-like behaviors in the healthy rats.
All the interest in the microbiome and stress has led to new insights for the treatment of stress-related behaviors through microbiome alteration. Allowing colonization with particular species of bacteria, for example Lactoacillus helveticus and Bifdobacterium longum in both rats and humans, has been shown to reduce psychological distress. Additionally, treatment with oligosaccharides, such as fructo-oligosaccharide or galacto-oligosaccharide in mice, can have anti-depressant effects by allowing different populations of microbes to thrive on these nutrient sources.
While the microbiome still holds many mysteries, we are beginning to understand just how important its functions are in our every day lives. So the next time you're feeling stressed out, you might want to check in with your microbes and see how they're feeling. They may play a critical role in getting you through this stress and on with your life without allowing long-term health complications, like depression, anxiety disorders, or inflammatory bowel disease, to develop. The best ways to keep a healthy microbiome are to eat a healthy diet, with emphasis on fibers that support the development of beneficial bacteria, take probiotics, stay physically active, and avoid antibiotics whenever possible. Or, simply change your lifestyle to reduce your stress level. However it is achieved, a healthy microbiome can be a great advantage in the years ahead.
The microbiome is the group of microbes (bacteria, fungi, archaea, etc.) that colonize a particular area of the body. The gut microbiome has been a population of intense research for quite a few years now. With increasing technology, we've begun to learn more and more about this population. We know that at homeostasis, each person has a specific microbial population that makes up their gut microbiome. This helps keep us healthy, keeping our digestive system running smoothly and ensuring successful processing of the food we eat. But in times of disease or stress, the microbiome in the gut can change dramatically, causing symptoms from loss of appetite to ulcers.
How exactly does stress change what's living in your intestines? It's all because of the gut-brain communications. This is achieved through neural projection pathways, neuroendocrine signaling, and entero-endocrine signaling, among other pathways. Your body uses messaging through the neurons and small chemical messengers in the form of endocrine signals to communicate over long distances, such as from the brain to the gut. In return, the gut uses the same mechanisms to send feedback to the brain. The microbes in the gut, however, can also produce chemical messages that get sent to the brain. When your body experiences stress, different chemical signals are sent to the gut; this can, in turn, cause some microbes to die and allow other microbes to take their place. The change in the composition of microbes can then change the chemical signals sent back to the brain. In this way, there is an intimate connection between the signalling input from the brain and the feedback the brain receives. These signals are essential to support mood, higher cognitive function, and behavior.
Work in animal models has highlighted the influence of stress on the gut microbiome and the subsequent influence of the microbiome on behaviors. For example, when mice are raised in a germ-free environment (no microbiome), the mice show reduced anxiety-like behaviors as compared to mice that are not raised in a germ-free environment. It has also been shown that the microbiome of the mice has an influence on the development of the amygdala, a region of the brain that plays a vital role in controlling behavioral and physiological responses to stress stimuli. The germ-free mice showed increased amygdala volume, suggesting an increased ability to handle stress. Additional studies have found that early-life stress can change an animal's microbiome for the rest of its life and that microbiome transplantation from depressed rats to healthy rats can lead to anxiety-like behaviors in the healthy rats.
All the interest in the microbiome and stress has led to new insights for the treatment of stress-related behaviors through microbiome alteration. Allowing colonization with particular species of bacteria, for example Lactoacillus helveticus and Bifdobacterium longum in both rats and humans, has been shown to reduce psychological distress. Additionally, treatment with oligosaccharides, such as fructo-oligosaccharide or galacto-oligosaccharide in mice, can have anti-depressant effects by allowing different populations of microbes to thrive on these nutrient sources.
While the microbiome still holds many mysteries, we are beginning to understand just how important its functions are in our every day lives. So the next time you're feeling stressed out, you might want to check in with your microbes and see how they're feeling. They may play a critical role in getting you through this stress and on with your life without allowing long-term health complications, like depression, anxiety disorders, or inflammatory bowel disease, to develop. The best ways to keep a healthy microbiome are to eat a healthy diet, with emphasis on fibers that support the development of beneficial bacteria, take probiotics, stay physically active, and avoid antibiotics whenever possible. Or, simply change your lifestyle to reduce your stress level. However it is achieved, a healthy microbiome can be a great advantage in the years ahead.
Labels:
archaea,
bacteria,
fungi,
microbiome,
probiotics,
stress
Sunday, April 23, 2017
Introverts, Assemble!
On Saturday, April 22, the March for Science took place at locations across the United States and the world. I had the good fortune to attend the flagship march in Washington, D.C. From talks and music on the main stage to teach-in tents across the Washington Monument lawn to the march itself, the day was quite an experience for me. I don't normally write personal blog posts, but it seems appropriate for this month to do just that.
 I attended the march with a few fellow biomedical science PhD students and an engineer. I know many of my other biomedical science friends were there as well. But this march wasn't just about biomedical scientists. It was about geologists and mathematicians and chemists and botanists. It was about our past and the present and the world we want to leave behind. It was about coming together from all scientific fields, from competing labs and engineering firms, from all over the globe. It was about making the facts known and showing the world that science has always held and will always hold great value for our society.
I attended the march with a few fellow biomedical science PhD students and an engineer. I know many of my other biomedical science friends were there as well. But this march wasn't just about biomedical scientists. It was about geologists and mathematicians and chemists and botanists. It was about our past and the present and the world we want to leave behind. It was about coming together from all scientific fields, from competing labs and engineering firms, from all over the globe. It was about making the facts known and showing the world that science has always held and will always hold great value for our society.
 I attended the march with a few fellow biomedical science PhD students and an engineer. I know many of my other biomedical science friends were there as well. But this march wasn't just about biomedical scientists. It was about geologists and mathematicians and chemists and botanists. It was about our past and the present and the world we want to leave behind. It was about coming together from all scientific fields, from competing labs and engineering firms, from all over the globe. It was about making the facts known and showing the world that science has always held and will always hold great value for our society.
I attended the march with a few fellow biomedical science PhD students and an engineer. I know many of my other biomedical science friends were there as well. But this march wasn't just about biomedical scientists. It was about geologists and mathematicians and chemists and botanists. It was about our past and the present and the world we want to leave behind. It was about coming together from all scientific fields, from competing labs and engineering firms, from all over the globe. It was about making the facts known and showing the world that science has always held and will always hold great value for our society.
I was amazed by the diversity of the people there. Not only were people diverse in interest, background, and occupation, but they were also diverse in ways you don't typically expect in the sciences. There were young children and senior citizens and everything in between. There were long-time career scientists and Nobel laureates and undergraduates just starting out. There were Christians and Muslims and atheists and agnostics. There were Republicans and Democrats and Independents. Men and women from all walks of life were gathered together to advocate scientific awareness.
While the message at times turned political, the overwhelming push from the people was for bipartisan, wholehearted support for the sciences. Acceptance of "alternative facts" and statements without data cannot continue to suppress the great advances being made every day in science and technology. Some emphasized the dangers of budget cuts to the National Institutes of Health and other scientific agencies, while others focused on the frightening state at the Environmental Protection Agency. Still others expressed concern for the uninformed American public and policy makers and the great number of sources of unreliable and untrue "scientific" information that create a mistrust of science.
While the message at times turned political, the overwhelming push from the people was for bipartisan, wholehearted support for the sciences. Acceptance of "alternative facts" and statements without data cannot continue to suppress the great advances being made every day in science and technology. Some emphasized the dangers of budget cuts to the National Institutes of Health and other scientific agencies, while others focused on the frightening state at the Environmental Protection Agency. Still others expressed concern for the uninformed American public and policy makers and the great number of sources of unreliable and untrue "scientific" information that create a mistrust of science.
As the march itself progressed from the Washington Monument to the Capitol Building, signs of all shapes, sizes, and recyclable materials braved the steadily increasing rain. Many gave a nod to the fact that it was Earth Day and featured images of Mother Earth, saying, "I'm with her." Others cleverly incorporated elements from the periodic table to spell out their messages. Still others pointed out how truly desperate the situation of science in America must be with phrases like, "It's so bad even the introverts are here." "Science not silence" and "Make America Smart Again" were other common sentiments. Neil deGrasse Tyson was frequently cited for his quote, "The good thing about science is that it's true whether or not you believe in it." Though many signs suffered from the elements, the spirit of their creators remained undeterred as we progressed along Constitution Avenue. Cheers and chanting were frequent throughout the crowd, as was reflective discussion among the participants.
The march was, in my opinion, a smashing success. It was a giant nerd convention with the outreach capabilities to include anyone and everyone. The breadth of scientific topics and passions highlighted throughout the event was mind-blowing. We often get so focused on our narrow sliver of the scientific community that it's easy to forget about the incredible power of all of us united. It was truly humbling to see the incredible amount of support for the sciences that exists in our country. We can only hope that the policies of the government will reflect that support moving forward. Recognition of the importance of science will not only lead to funding assurance to support vital research, but it will also ensure that we continue to make the technological advances to improve the health and lives of everyone across the globe. Science saves lives, and science paves the future.
Friday, March 31, 2017
Mobile phones can make the difference for infectious disease management
Imagine going to the doctor for an HIV test in sub-Saharan
Africa. You will likely have to travel a long distance to get to a clinic and,
once you arrive, the HIV test will require specimens to be sent to a central
testing laboratory for the actual test to be performed. You will then have to
wait for the results to be delivered to the clinic and travel back to get the
diagnosis. In Zambia, it takes approximately 92 days for this process to be
completed. When you are an HIV-positive mother waiting to find out if your
newborn child needs anti-retroviral therapy, each of those 92 days can be the
difference between life and death for your baby.
New work from Dr. William Moss of the Johns Hopkins Bloomberg School of Public Health and his group focuses on finding ways to
shorten the 92-day wait for HIV test results. Capitalizing on the increasing
amount of technology available in rural Zambia, Moss and colleagues conducted a
study using text messaging to deliver the HIV test results directly to mothers
or to the rural health clinics from the central testing lab. They found that by sending a text message to the mother directly,
they could reduce the time from sample collection to receipt of diagnosis to
just 18 days. Unfortunately, the use of mobile phones is still not widespread;
only 30% of mothers in the study had ever used a mobile phone. Luckily, the
local clinics do have mobile phones, so using text messaging to deliver results
to the clinic can still decrease the time to diagnosis to 36 days. By cutting
56 days off the wait for the diagnosis, the HIV-positive children will receive
anti-retroviral therapy nearly 2 months earlier. Reducing the time to treatment
for HIV-positive children has been shown to significantly reduce the
HIV-associated morbidity and mortality, vastly improving their quality of life.
This is not the first time mobile phone technology has been
used in Africa to impact health care. As the number of cell phones throughout
the area has increased, some clinics have chosen to provide expectant mothers
with a mobile phone so that they can get expedited access to an experienced
midwife or healthcare professional. In other areas across the continent, SMS services have been used to target pregnant women and new mothers to ensure early detection of life-threatening emergencies and adherence to treatment regimens for pathogen clearance. These methods and Moss’s study highlight
the same conclusion: increased access to mobile phones throughout the area can have
major positive impacts on health care. Funding and infrastructure for such
technological advances remains a major issue hindering progress.
As noted by Moss in his recent BMC
Pediatrics paper, implementation of the mobile phone messaging system for
test result delivery was by no means trivial. It required the hiring and
training of new staff members, along with the purchase of the study’s mobile
phone and “talk time” for the phone. However, with the right funding and
infrastructure, the spread of technology throughout Africa has the potential to
have significant and widespread impacts on healthcare. Studies such as the one
by Moss and colleagues highlight the great potential of available technology.
This encourages governments and private sector investors to take note and get
involved. Someday soon, nationwide mobile phone messaging systems may become
common place, drastically cutting the time to diagnosis and improving
individual’s access to care and treatment.
Wednesday, February 15, 2017
Killing two birds with one stone: A new vaccine to fight rabies and MERS-Cov
In 2003, the world was faced with a serious biological threat. The severe acute respiratory syndrome (SARS) virus hit the scene in China and quickly spread to 28 countries across the globe. The reason for the rapid and global spread lay in the very nature of the virus' infection. It can be spread by close person-to-person contact through respiratory droplets produced when an infected person sneezes or coughs, and the initial symptoms of disease are very non-specific. This allowed the virus to easily be carried via international travel throughout the world. While the number of cases quickly rose to a total of 8,098 within 6 months, the global response was also rapid. The World Health Organization quickly activated their global alert system and began aiding countries in identifying and quarantining those infected and at risk. Thanks to this global response, SARS was quickly handled. However, this virus showed the world how at-risk we are to respiratory viruses in an age of increasing global travel.
Since the 2003 SARS outbreak, scientists have been on the look-out for the SARS virus and other related viruses in an attempt to minimize outbreaks. These viruses are part of a family known as Coronaviridae, specifically the coronavirus sub-section of this family (a typical virion is shown to the right). They are single-stranded, positive sense RNA viruses, which means that as soon as the virus invades a host cell, it can begin making its own proteins and progeny immediately without the need for time for replication or transcription of the genetic material. The coronaviruses that cause respiratory syndromes infect the cells of the lungs, leading to severe and sometimes deadly pneumonia.
In 2012, a novel coronavirus was identified in Saudi Arabia; it was named the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). It quickly spread to the United Kingdom through travel. While the MERS-CoV has never caused an outbreak as large as that of the SARS virus, it has also not been contained as successfully. MERS-CoV outbreaks have continued to pop up from 2012 to now, with the most recent outbreak update coming just last week from Saudi Arabia. The MERS-CoV outbreaks have caused a total of 1,905 confirmed cases in 27 countries, with 677 deaths. The severe pneumonia caused by MERS-coV is more deadly than that caused by SARS and other coronoviruses, leading to the alarmingly high 37% death rate.
There are currently no vaccines and no treatments for MERS-CoV. Quarantining those infected and using additional precautions when treating these patients have been the only successful preventative measures to reduce spread. The biggest problem for complete elimination of this virus is that, unlike the SARS virus, the MERS-CoV can also infect an animal that has frequent contact with humans: camels. In many parts of the globe, camels are essential for transportation and play a pivotal role in the economy. Since camels have been shown to be a reservoir for the MERS-CoV, and people in these regions need to continue to have close contact with these animals, the virus has an easy route to re-enter the human population even with the implementation of the same control measures that were so successful with the SARS virus.
Recent work has focused on elimination of virus from the camel population as a course of action to reduce human infections. Starting with a rabies vaccine, which has long been given to animals and is well-tolerated, a group in the United States has shown that immunity to both rabies and MERS-CoV can be achieved in mice. In order to do this, they took a piece of the MERS-CoV spike protein and fused it to the rabies G protein. This allowed a portion of the MERS-CoV to be incorporated into the rabies virus vaccine particles for delivery to the mice. After receiving the immunization, mice were challenged with the MERS-CoV and were found to be protected from infection. The researchers also found high levels of neutralizing antibodies against both MERS-CoV and the rabies virus in the blood of the mice.
While this vaccine candidate is still in the early stages of development, the successful use of the previously tested and approved rabies vaccine as a backbone may provide a way to shorten the timeline to implementation of the vaccine for animals on a larger scale. This could provide a way to start to eliminate the MERS-CoV reservoir and begin to reduce outbreaks in people across the globe. While other research groups are still searching for human vaccination and treatment strategies that will greatly improve our ability to decrease disease severity and save lives, dealing with this large camel reservoir will be an essential step before disease elimination and eradication can truly be considered.
Since the 2003 SARS outbreak, scientists have been on the look-out for the SARS virus and other related viruses in an attempt to minimize outbreaks. These viruses are part of a family known as Coronaviridae, specifically the coronavirus sub-section of this family (a typical virion is shown to the right). They are single-stranded, positive sense RNA viruses, which means that as soon as the virus invades a host cell, it can begin making its own proteins and progeny immediately without the need for time for replication or transcription of the genetic material. The coronaviruses that cause respiratory syndromes infect the cells of the lungs, leading to severe and sometimes deadly pneumonia.
In 2012, a novel coronavirus was identified in Saudi Arabia; it was named the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). It quickly spread to the United Kingdom through travel. While the MERS-CoV has never caused an outbreak as large as that of the SARS virus, it has also not been contained as successfully. MERS-CoV outbreaks have continued to pop up from 2012 to now, with the most recent outbreak update coming just last week from Saudi Arabia. The MERS-CoV outbreaks have caused a total of 1,905 confirmed cases in 27 countries, with 677 deaths. The severe pneumonia caused by MERS-coV is more deadly than that caused by SARS and other coronoviruses, leading to the alarmingly high 37% death rate.
There are currently no vaccines and no treatments for MERS-CoV. Quarantining those infected and using additional precautions when treating these patients have been the only successful preventative measures to reduce spread. The biggest problem for complete elimination of this virus is that, unlike the SARS virus, the MERS-CoV can also infect an animal that has frequent contact with humans: camels. In many parts of the globe, camels are essential for transportation and play a pivotal role in the economy. Since camels have been shown to be a reservoir for the MERS-CoV, and people in these regions need to continue to have close contact with these animals, the virus has an easy route to re-enter the human population even with the implementation of the same control measures that were so successful with the SARS virus.
 |
| The MERS-CoV can be spread from camels to humans in many ways. |
While this vaccine candidate is still in the early stages of development, the successful use of the previously tested and approved rabies vaccine as a backbone may provide a way to shorten the timeline to implementation of the vaccine for animals on a larger scale. This could provide a way to start to eliminate the MERS-CoV reservoir and begin to reduce outbreaks in people across the globe. While other research groups are still searching for human vaccination and treatment strategies that will greatly improve our ability to decrease disease severity and save lives, dealing with this large camel reservoir will be an essential step before disease elimination and eradication can truly be considered.
Subscribe to:
Posts (Atom)

